
Pega Pharmacovigilance Software
Improve speed, compliance and risk management for drug and device safety

"We’ve been able to centralize the tracking of exchange of adverse events, aggregate reports, and safety signals with our partners in our global group. That was only made possible by having the efficiencies and the automation that the system brought."
Get to know Pega Pharmacovigilance Software
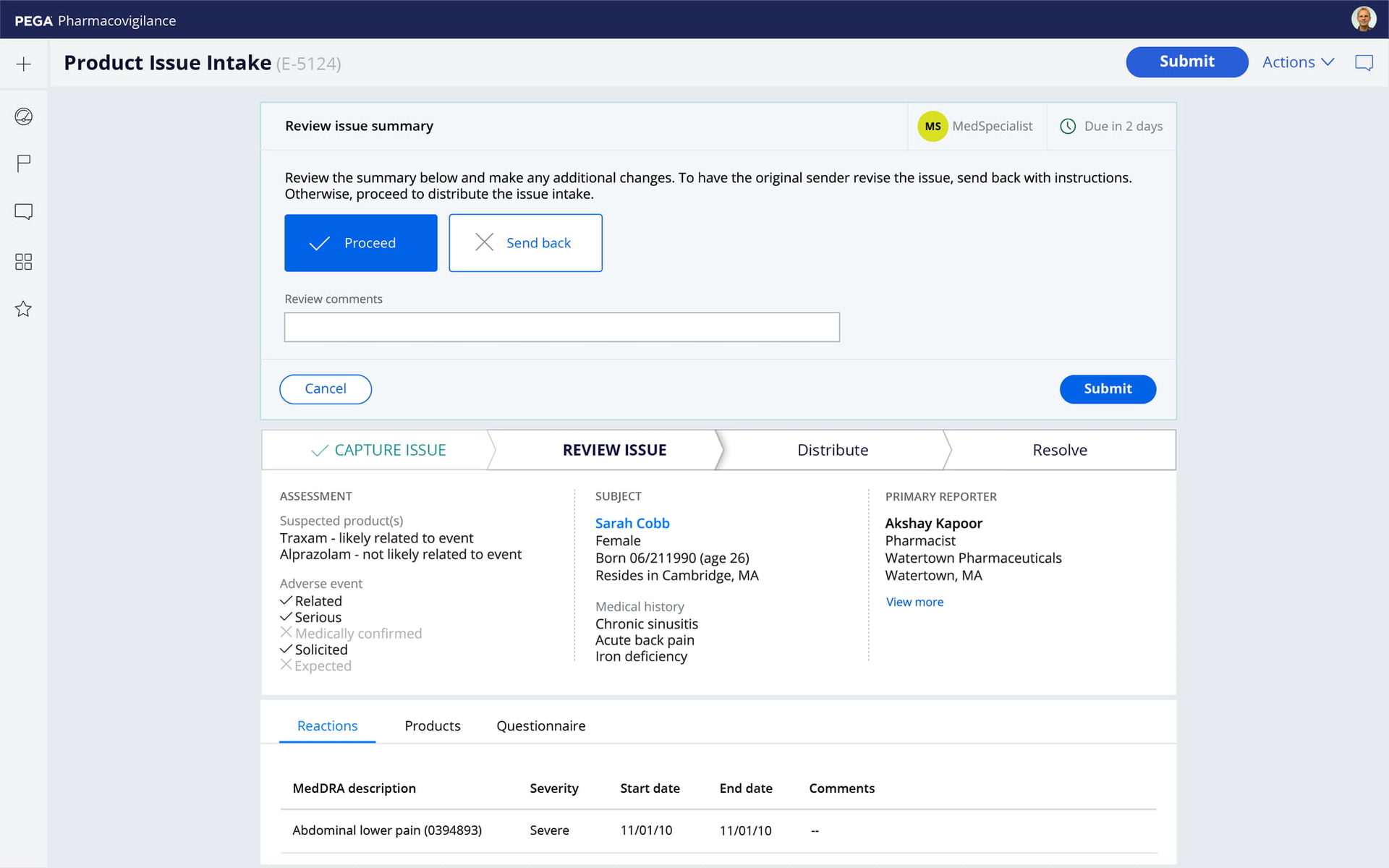
Dynamic intake
Pega features responsive, multi-channel intake that automatically adapts to products, regions, and risk profiles. The result: Faster intake and higher quality data, for fewer follow-ups. Manage all AEs and Complaints, for any product or source, with clinical, social, and contact center support.
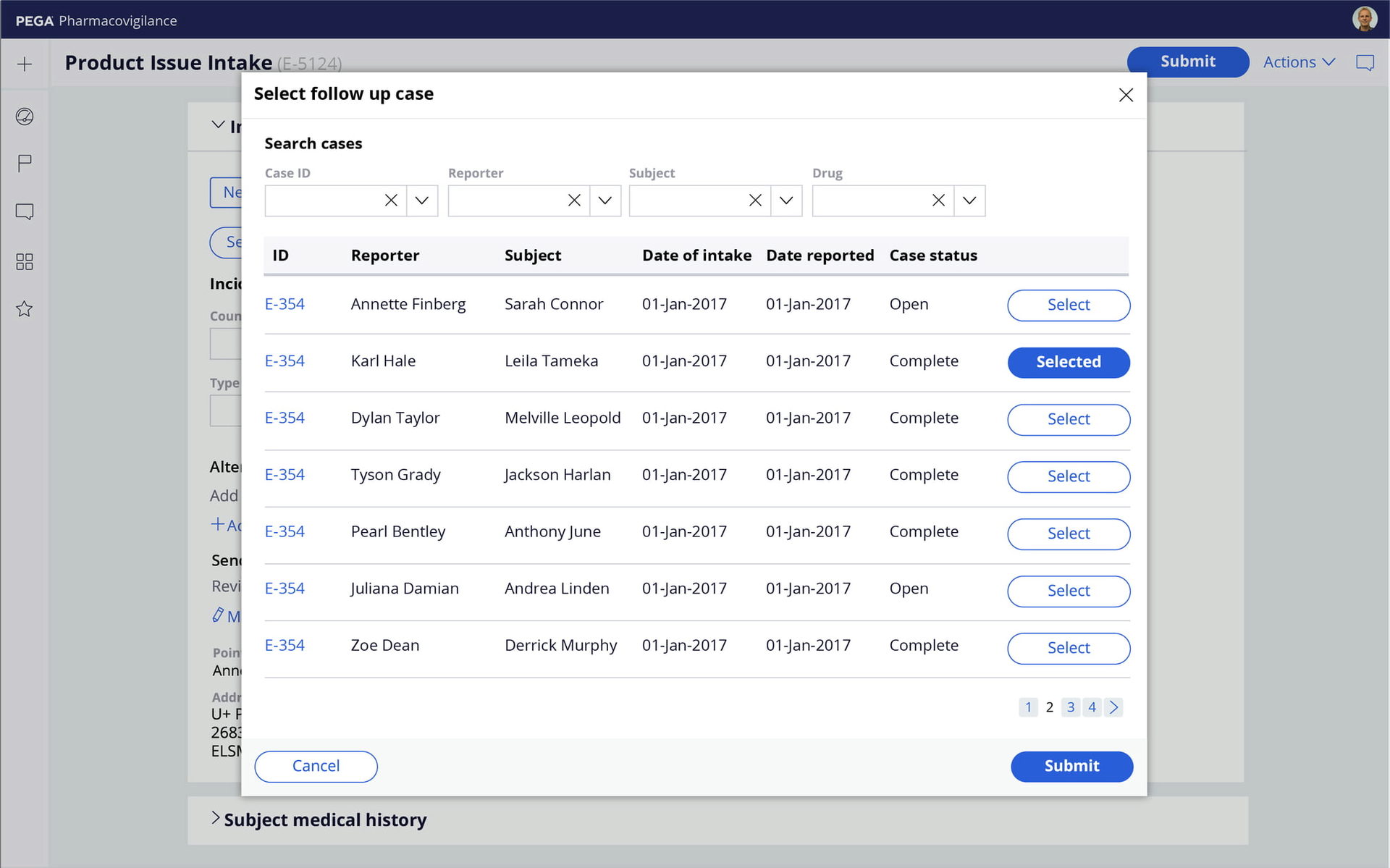
Automated processes
Accelerate and scale individual and bulk AE case processing with risk-profile-driven assessments and MedDRA coding. Manage affiliate workflows and global submissions, generate MedWatch, CIOMS, E2B M2/R3, and eMDR reports, and ensure data quality with built-in Part 11 audit trails and validations.
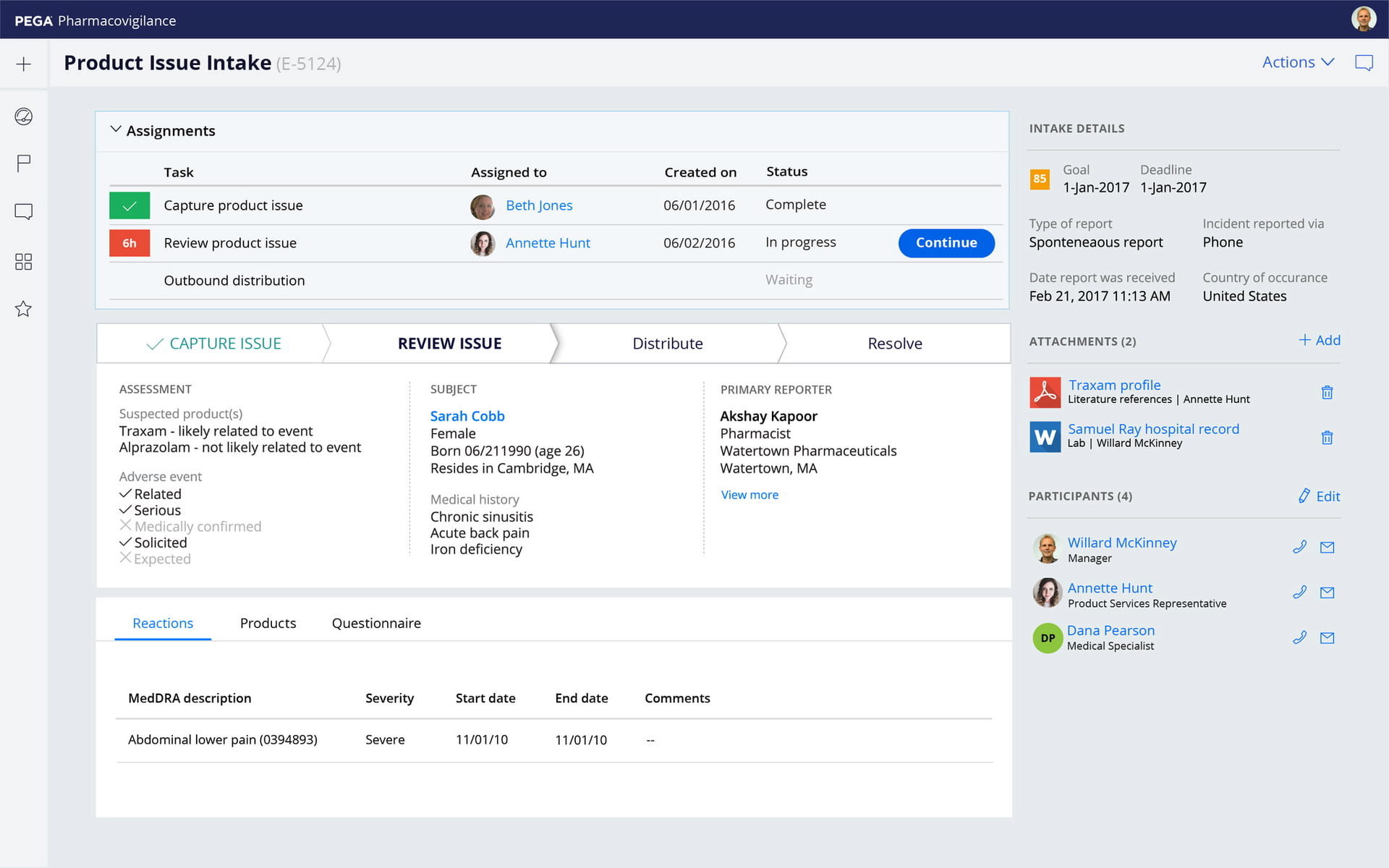
Expanded visibility
Gain transparency and automation for your entire safety ecosystem. With Pega you can connect social media channels and integrate Natural Language Processing and Optical Character Recognition into your processes. Track partner activities, including Pharmacovigilance Agreements and cases exchange with partners.

Wrap and renew
Pega leverages your existing investments, helping you improve the efficiency of your AE intake and work seamlessly with your existing safety system. Readily support and easily extend the latest E2B submission formats, and provide full transparency with automated case status tracking for electronic submissions.

Pharma and med device support
Harmonize and align operations across product types with support for AEs and product complaints for drugs, vaccines, biologics, and med devices. Automate submissions processes for E2B, eMDR, MedWatch, and CIOMS. Tailored interfaces for different product types make case processing more efficient.
Additional Product Features
Pega BPM and Case Management
Quickly configure business applications with built-in business process and case management.
Data Access Made Easy
Create a virtualized application data layer with unique data management capabilities.
Pega Cloud
Fetch customer data from different sources, in real time, while preserving data integrity.
Customer Engagement for Life Sciences
Move beyond a transactional, data-centric view of patients and healthcare professionals.
Life Sciences Industry Foundation
Accelerate the delivery of life sciences applications using the Pega platform
Customer Case Studies
See how customers are using Pega pharmacovigilance software to transform their enterprises.

Case Study
